Magnesia-carbon bricks are primarily used in the construction of converter furnace linings. Different types of magnesia-carbon bricks are selected for different parts of the converter, depending on the specific location and the corrosive conditions, allowing for a comprehensive lining design.
For vulnerable areas such as the trunnion and slag line, which are frequently subjected to airflow and slag erosion, high-grade magnesia-carbon bricks with a carbon content of 16% to 18% and added metal antioxidants are used to enhance their corrosion resistance.
On the charging side, due to frequent scouring by molten slag and mechanical stress, high-grade magnesia-carbon bricks with a carbon content of 10% to 14% and added metal antioxidants are used to enhance their impact resistance and slag coating protection, extending the lifespan of the furnace.
In the furnace hood area, medium-grade magnesia-carbon bricks with a carbon content of 14% to 16% are used to reduce carbon vaporization, increase the tightness of the lining, and prevent accidents such as brick fall and steel leakage.
In the molten pool area, which is the main body of the converter, the refractory lining is constantly subjected to erosion from molten steel and the effects of gas flow. Therefore, high-grade magnesia-carbon bricks with a carbon content of 16% to 18% and added metal antioxidants are used for construction.
At the furnace bottom, medium-grade magnesia-carbon bricks with a carbon content of 14% to 16% and without metal additives are used. In short, the use of magnesia-carbon bricks in converters is primarily for constructing the furnace body, implementing a comprehensive refractory lining strategy to extend the furnace life and improve the converter’s service life.
Damage Mechanisms of Magnesia-Carbon Bricks
1. The Destruction Process of Magnesium Carbon Bricks
The deterioration of magnesium carbon bricks primarily results from carbon oxidation within the bricks, forming a decarburized layer. Compounded by the significant disparity in thermal expansion coefficients between magnesium oxide and graphite at high temperatures (1.4% and 0.2% respectively at 1000°C), leading to structural loosening and reduced strength. Subsequently, erosion by molten slag and mechanical abrasion cause the magnesium oxide particles within the brick to gradually melt away, resulting in layer-by-layer detachment and ultimately the destruction of the magnesia-carbon brick. The degradation sequence of magnesia-carbon bricks is: oxidation → decarburization → loosening → erosion → abrasion → detachment → failure.
2. Failure Mechanism of Magnesium Carbon Bricks
Extensive research has demonstrated that above 1600°C, the following reaction is the primary cause of damage to magnesium-carbon bricks: MgO(s) + C (s) → Mg(g) + CO(g) The deterioration of magnesium-carbon bricks begins with the oxidation of carbon in the hot face of the working lining, forming a thin decarburized layer. This carbon oxidation results from continuous oxidation by iron oxides in the slag, atmospheric oxygen (O₂), and other oxides like CO₂, SiO₂, as well as the vaporization effect of MgO dissolved in molten steel or within the bricks. Second, high-temperature molten slag infiltrates the pores within the decarburized layer or cracks generated by thermal stress. There, it reacts with the magnesium oxide in the bricks to form low-melting-point compounds. This causes a qualitative change and weakening of the brick’s surface layer. Under the combined stresses of vigorous steel-slag agitation and mechanical erosion, the surface layers progressively peel away, leading to the failure of the MgO-carbon bricks. This cycle repeats, causing the furnace lining to thin layer by layer, ultimately necessitating furnace repairs, maintenance, or shutdown.
(1) The deterioration of carbon-magnesia bricks primarily results from the oxidation of carbon within the bricks. This oxidation occurs through the following reaction:
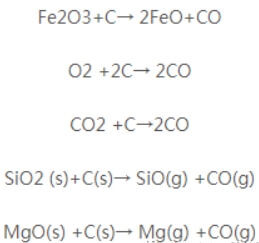
The oxidation of carbon disrupts the carbon network structure within the bricks, resulting in a loosened microstructure and reduced product strength. Simultaneously, it increases porosity, thereby accelerating the erosion of the bricks by furnace slag.
(2) The Impact of Pores Pores in magnesia-carbon bricks, particularly open pores, significantly influence their deterioration. During use, these pores primarily facilitate carbon oxidation damage, thereby accelerating slag erosion of the brick lining and ultimately causing brick failure. Open pores within the bricks draw in external air during cooling. During reheating, oxygen in the air reacts with surrounding carbon to produce CO, which is expelled. This cyclical process repeatedly increases porosity. Additionally, the binder present in magnesium carbon bricks is a key factor in pore formation. Phenolic resin is commonly used as the binder for these bricks. Adding 3%–4% phenolic resin results in a relatively low porosity of approximately 3% after forming. However, during use, the phenolic resin decomposes upon heating, releasing gases such as H₂, H₂, CH₄, CO, and CO₂. These gases evaporate and escape, forming pathways that create pores and further increase porosity. Consequently, oxygen in the air and oxides in the slag erode the bricks through these pores. This process accelerates the oxidation and degradation of carbon while intensifying the reaction between slag and MgO within the bricks, leading to the deterioration of magnesia-carbon bricks. This cycle repeats continuously. Through carbon oxidation and slag erosion, the carbon network structure within the bricks is disrupted, leading to a loose microstructure and reduced high-temperature strength. Simultaneously, low-melting-point compounds form on the brick surface, weakening and altering its properties. Under the combined stresses of vigorous slag agitation, mechanical abrasion, and thermal shock, the bricks gradually delaminate layer by layer, ultimately causing their failure.
Countermeasures
1. High-quality fused magnesia is used as raw material. This premium fused magnesia features well-developed crystalline structure, large grain size, high bulk density, low chemical reactivity, and excellent corrosion resistance. It also exhibits strong resistance to carbon reduction, effectively preventing erosion of MgO particles. Secondly, it possesses high magnesium oxide content and low impurity levels, particularly low SiO₂ content. The minimal silicate phase in its structure inhibits reactions with graphite in the matrix, preventing carbon oxidation. Furthermore, its high degree of direct crystal bonding avoids liquid phase formation at grain boundaries at high temperatures, thereby preventing erosion and blocking slag penetration. Given the higher cost of premium fused magnesia, production should incorporate sintered magnesia based on the specific application areas of magnesia-carbon bricks in converters. Specifically, premium fused magnesia should be used for high-wear zones, while sintered magnesia is suitable for areas subject to less severe damage.
2. Enhancing Graphite Purity Graphite is the element with the highest known melting point, reaching 3,500°C. It exhibits a low thermal expansion coefficient and excellent chemical stability. Graphite purity significantly impacts the performance of magnesia-carbon bricks. As graphite purity increases, the erosion index of magnesia-carbon bricks decreases sharply, while the high-temperature flexural strength index improves markedly. Graphite with a carbon content exceeding 95%, preferably over 98%, is generally required. This is because higher graphite purity reduces ash content and minimizes SiO₂ inclusion. At elevated temperatures, SiO₂ oxidizes carbon within the brick, increasing porosity and structural loosening. It also forms low-melting compounds with MgO, Fe₂O₃, and other elements, accelerating the deterioration of magnesium-carbon bricks. Additionally, carbon’s high thermal conductivity and low expansion coefficient make it resistant to slag wetting. This enhances the product’s thermal shock resistance and prevents slag from penetrating the brick interior through pores.
3. Adding Metal Antioxidants
Magnesium carbon bricks have two primary weaknesses:
Poor oxidation resistance and low high-temperature strength. To overcome these weaknesses, adding appropriate amounts of metal antioxidants is one effective approach. This simultaneously enhances the product’s oxidation resistance and significantly improves its high-temperature strength.
Currently, commonly used metal antioxidants include metallic Al powder and Si powder. Metallic Al powder has a melting point of 659°C and exhibits a stronger affinity for oxygen than carbon, thereby inhibiting carbon oxidation. During use, as temperature rises, it progressively forms Al₄C₃, Al₂O₃, and MgO·Al₂O₃. The reaction equations 2 are as follows:
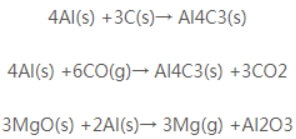
Among these, AI4C3 is a high-temperature phase that bridges magnesia and graphite particles, enhancing the product’s high-temperature strength. The formation of AI2O3 is accompanied by volumetric expansion, increasing the matrix density and improving oxidation resistance. The formation of magnesium aluminum spinel (Al2O3·MgO) introduces additional high-temperature phases into the product, thereby enhancing its high-temperature performance. Therefore, adding metallic aluminum powder enhances the oxidation resistance of magnesia-carbon bricks. Silicon powder has a greater affinity for oxygen than carbon. During use, silicon reacts with carbon to form silicon carbide (SiC), and reacts with MgO to form the high-melting-point compound 2MgO·SiO₂, accompanied by some volume expansion. This process inhibits the oxidation of carbon. The reaction equations 3 are as follows:
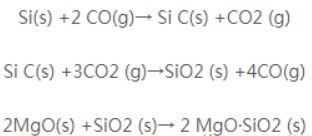
The formation of these reaction products often blocks channels or fills pores, preventing CO and CO₂ from migrating into the brick and thus protecting the carbon within from oxidation or preventing oxidation altogether. Simultaneously, they create new high-temperature phases that enhance the brick’s density and strength while forming a new protective layer resistant to oxidation and erosion. This inhibits carbon oxidation and improves the brick’s resistance to erosion.
4. Selection of Thermosetting Phenolic Resin as Binder
For magnesia-carbon bricks, selecting an optimal binder is critical. Due to the poor wettability of carbon materials, it is difficult to form high-strength composites with magnesia. Phenolic resin, however, possesses high fixed carbon content and excellent wetting properties. It uniformly distributes across the surfaces of MgO particles and graphite, firmly bonding magnesia and graphite particles. Furthermore, it forms a continuous carbon network structure at high temperatures, significantly enhancing the strength of magnesia-carbon bricks and improving the product’s resistance to erosion.
Conclusion
(1) The deterioration of magnesia-carbon bricks primarily results from carbon oxidation, forming a decarburized layer that causes structural loosening. Combined with high-temperature erosion from furnace slag and mechanical abrasion, this leads to layer-by-layer spalling and eventual failure of the bricks.
(2) Magnesium carbon bricks are manufactured using high-quality fused magnesia and graphite with a carbon content exceeding 98% as raw materials. Appropriate amounts of aluminum powder and silica powder (click link for product details) are added as antioxidants, with thermosetting phenolic resin selected as the binder.
